FABHALTA RESULTS
FABHALTA clinical study overview
The APPEAR-C3G trial included 74 adults with C3G who had never received a kidney transplant and studied the percentage reduction in urine protein-to-creatinine ratio (UPCR), a test used to measure protein in urine. Urine samples were collected over 24 hours, with results from the start of the study compared against those after 6 months. All patients were receiving a stable dose of maximally tolerated blood pressure medications (ACEi/ARB) with or without other background therapies for 90 days prior to starting the study and throughout the study.
Safety and effectiveness of FABHALTA® in patients with C3G following kidney transplant have not been established.
What Was Studied? (Primary Study Objective)
The reduction in UPCR, a test used to measure protein in the urine (proteinuria), after 6 months of treatment with FABHALTA or placebo.
ACEi, angiotensin-converting enzyme inhibitor; ARB, angiotensin receptor blocker; C3G, complement 3 glomerulopathy.
Clinical study results
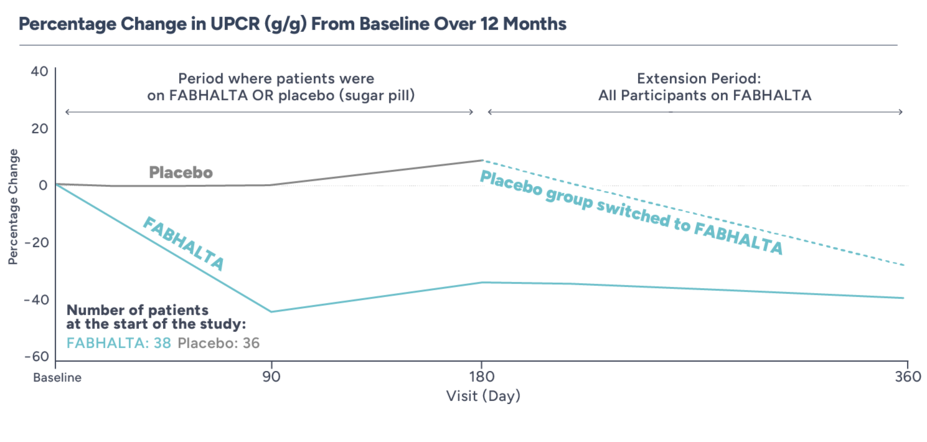
FABHALTA SUBSTANTIALLY REDUCED PROTEINURIA COMPARED TO PLACEBO AT 6 MONTHS
Clinical study details
The study included 74 adults with biopsy-confirmed C3G who had never received a kidney transplant. All patients had elevated proteinuria (UPCR ≥1.0 g/g) and eGFR ≥30 mL/min/1.73 m2 at the start of the study and never had a kidney transplant
For the first 6 months, 38 people received FABHALTA 200 mg twice daily and 36 people received placebo (sugar pill) twice daily
During the next 6 months (extension period), all participants took FABHALTA 200 mg twice daily
eGFR, estimated glomerular filtration rate.
Did you know?
Increasing levels of proteinuria over time is an important indicator that your C3G may be getting worse.
Ask your doctor how FABHALTA can help reduce proteinuria.
Ready to start the conversation with your doctor?
Doctor Discussion Guide
WITH FABHALTA, PROTEINURIA REDUCTION AT MONTH 6 WAS SUSTAINED THROUGH MONTH 12
In the study, a different measurement was taken to measure protein in the urine (proteinuria) called first morning void (FMV). FMV refers to the first urination of the day after you wake up and is more concentrated at this time.
Although there was a numerical improvement at month 6 between FABHALTA and placebo (sugar pill) in the reduction of proteinuria measured by FMV, this may not be attributable to FABHALTA alone and could be due to chance.