DISCOVER FABHALTA
FABHALTA: A first-of-its-kind targeted IgAN treatment
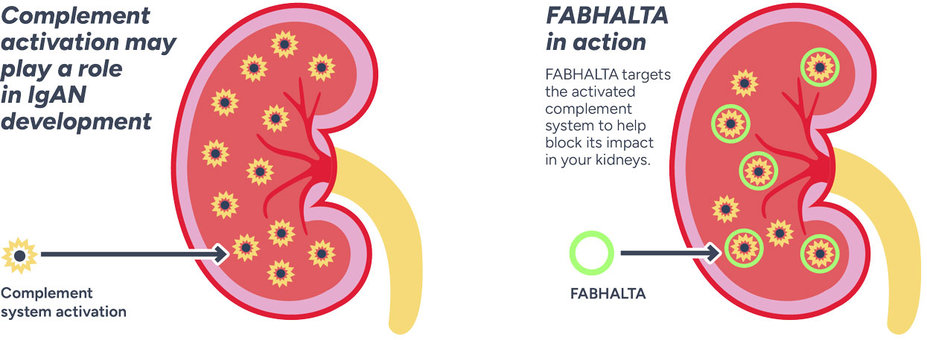
In immunoglobulin A nephropathy (IgAN), unwanted IgA antibodies build up in your kidneys, leading to the activation of multiple pathways including a part of your immune system called the complement system.
FABHALTA® is the first and only FDA-approved treatment for adults with primary IgAN at risk of their disease worsening quickly, that targets the complement system.
FABHALTA was studied in adults with IgAN
The APPLAUSE-IgAN trial is a phase 3 clinical study for adults with primary IgAN that studied the percentage reduction in urine protein-to-creatinine ratio, or UPCR, a test used to measure protein in urine. In this study, urine samples were collected over 24 hours, with results from the start of the study compared against those after 9 months.
Who was studied?
Patients with biopsy-proven IgAN with elevated proteinuria (UPCR ≥1g/g) who were on a stable dose of maximally tolerated blood pressure medications (ACEi/ARB) with or without other background therapies before and during the study.
How was the study done?
Efficacy results were analyzed after the first 125 patients taking FABHALTA (200 mg twice daily) and 125 patients taking placebo (sugar pill) received 9 months of study drug.
FABHALTA substantially reduced proteinuria in adults with primary IgAN at risk of their disease worsening quickly
Primary study objective
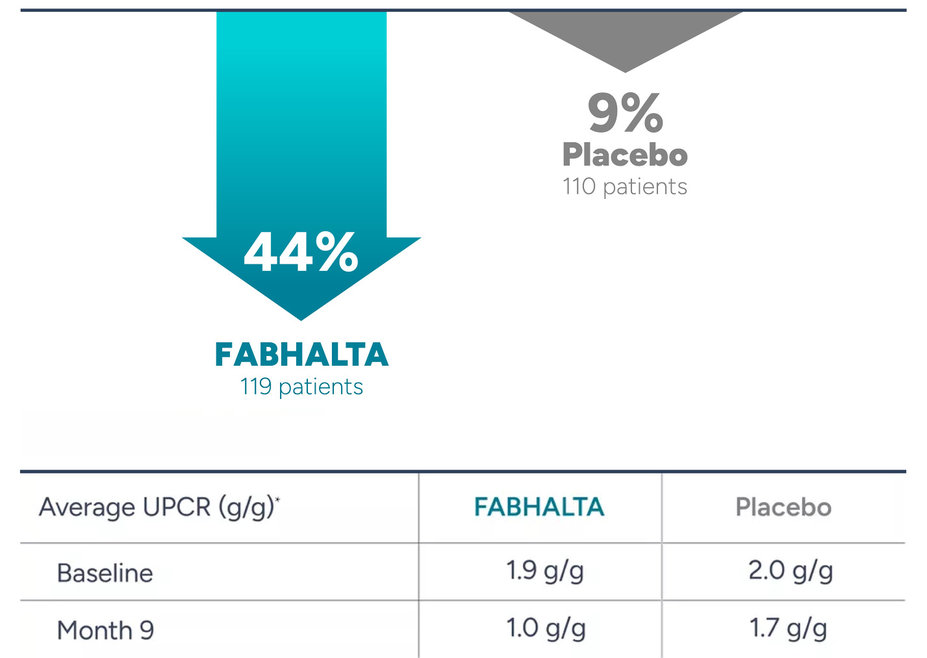
FABHALTA was shown to substantially reduce proteinuria levels at 9 months compared to placebo (sugar pill).
FABHALTA is used to reduce proteinuria in adults with primary IgAN at risk of their disease worsening quickly.
Proteinuria reduction at 9 months*
*Percent reduction was calculated by comparing average proteinuria levels at the start of the study and at 9 Months; Results for patients requiring rescue treatments for IgAN were assumed to relate to disease worsening. As of Month 9, 7 (5.6%) patients in the placebo (sugar pill) arm and 0 patients in the FABHALTA arm received rescue treatment for IgAN.
The most common adverse reactions reported by 5% or more of the people in the clinical trial included:
- Nasal congestion, runny nose, cough, sneezing, and sore throat (upper respiratory tract infection)
- Lipid disorder
- Pain in the stomach (abdomen)