What is the most important information to know about FABHALTA?
FABHALTA is a medicine that affects part of your immune system and may lower the ability of your immune system to fight infections.
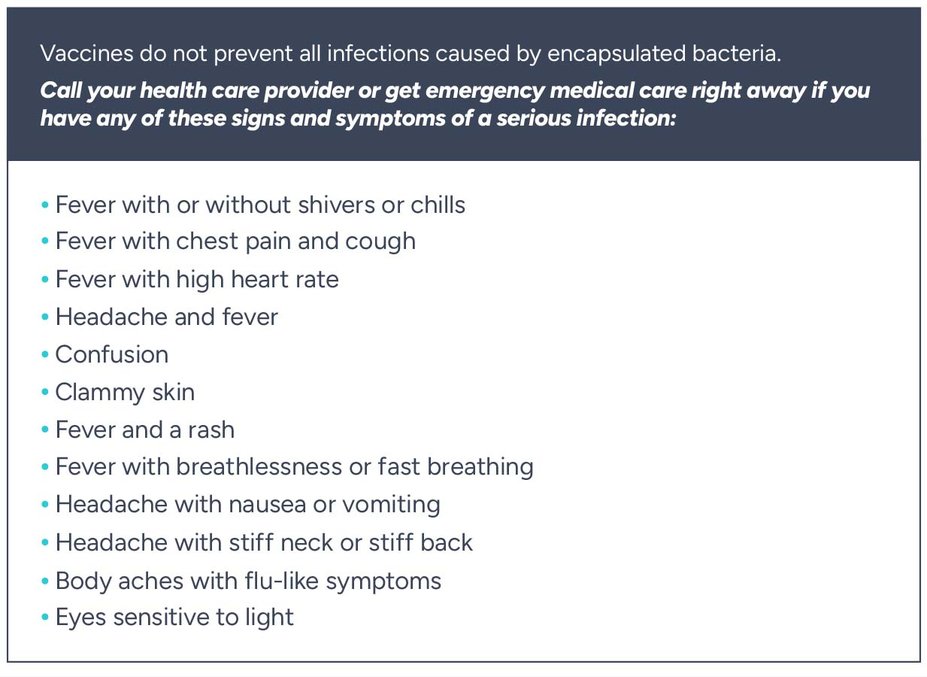
FABHALTA increases your chance of getting serious infections caused by encapsulated bacteria, including Streptococcus pneumoniae, Neisseria meningitidis, and Haemophilus influenzae type b. These serious infections may quickly become life-threatening or fatal if not recognized and treated early.
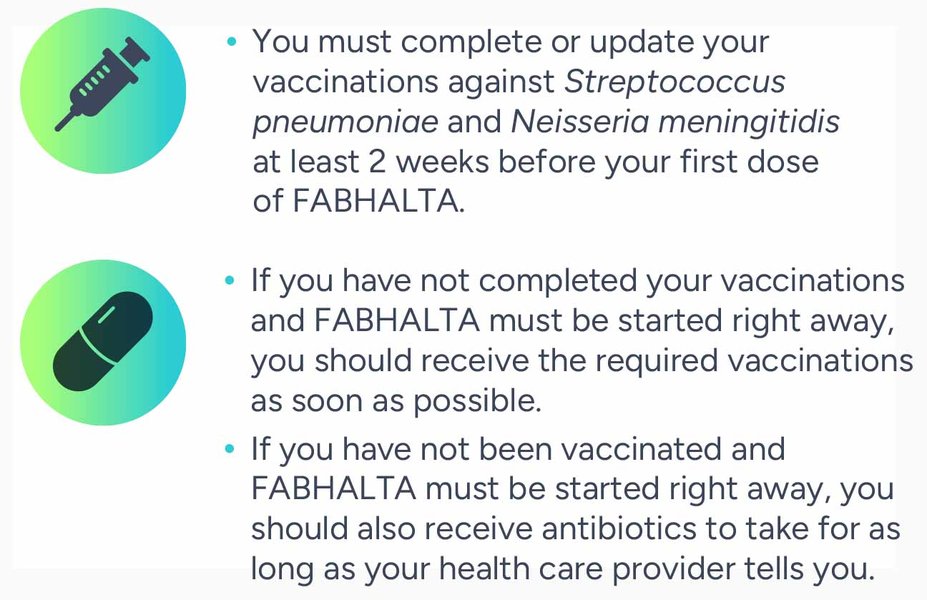
If you have been vaccinated against these bacteria in the past, you might need additional vaccinations before starting FABHALTA. Your doctor will decide if you need additional vaccinations.
Because of the risk of serious infection caused by encapsulated bacteria, FABHALTA is only available through a Risk Evaluation and Mitigation Strategy (REMS) program that requires vaccinations.
While taking FABHALTA, you should be revaccinated according to current medical guidelines for encapsulated bacteria.
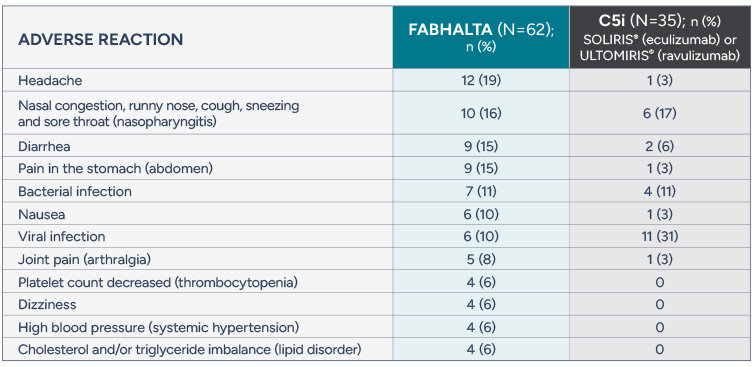
Safety profile of FABHALTA in C5i treatment-experienced adults with PNH
Adverse reactions reported in >5% of adults with PNH treated with FABHALTA (24-week treatment period)
Tell your doctor about any side effect that bothers you or that does not go away. These are not all the possible side effects of FABHALTA.
Serious adverse reactions (kidney infection, urinary tract infection, and COVID-19) were reported in 2 people (3%) with PNH receiving FABHALTA
FABHALTA may increase your cholesterol and triglycerides and your health care provider will do blood tests to check them periodically during treatment
Rash was reported in two people (3%) taking FABHALTA
No patient discontinued FABHALTA or a C5i due to an adverse reaction during the 24-week clinical trial. One patient in the clinical trial discontinued FABHALTA due to pregnancy
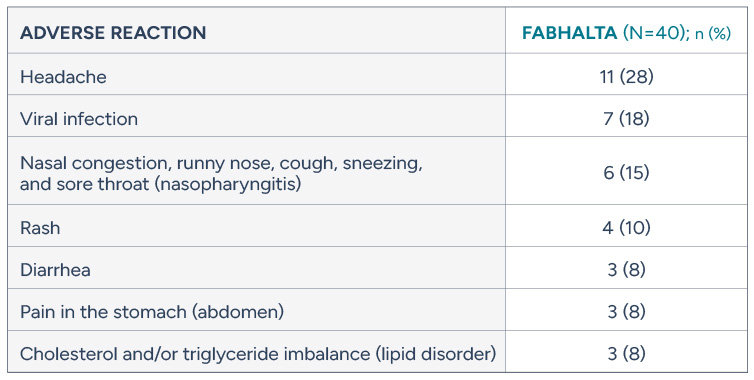
Safety profile of FABHALTA in complement-inhibitor naive adults with PNH
Adverse reactions reported in >5% of adult patients treated with FABHALTA (24-week treatment period)
Tell your doctor about any side effect that bothers you or that does not go away. These are not all the possible side effects of FABHALTA.
Serious adverse reactions (COVID-19 and bacterial pneumonia) were reported in 2 people (5%) with PNH receiving FABHALTA
FABHALTA may increase your cholesterol and triglycerides and your health care provider will do blood tests to check them periodically during treatment.
Nausea and bacterial infection were each reported in two people (5%) and dizziness and hives were each reported in one person (3%)
No patient discontinued FABHALTA due to an adverse reaction in the 24-week clinical trial
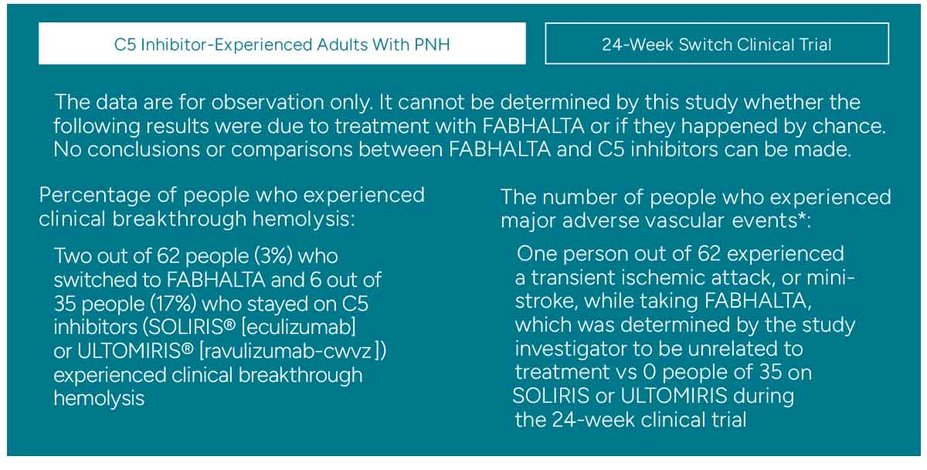
Clinical breakthrough hemolysis and major adverse vascular events observed with FABHALTA
Clinical breakthrough hemolysis was defined in the study as a person who experienced a decrease of ≥2 g/dL in hemoglobin compared to the last assessment or within 15 days, or other significant increase in PNH-related signs or symptoms. People were also screened for other lab criteria
Major adverse vascular events were defined in the study as those involving the blood vessels such as stroke, heart attack, and blood clots
ULTOMIRIS (ravulizumab-cwvz) and SOLIRIS (eculizumab) are registered trademarks of Alexion Pharmaceuticals, Inc.